Digitalized Drug Forecasting Minimizes Waste In Clinical Trial Supply Chain
By Kristel Rens, director, business transformation & innovation, clinical supply chain, J&J Innovative Medicine

In today's economic landscape, environmental sustainability has gained significant attention, leading to a focus on reducing CO2 emissions and greenhouse gases in supply chains through energy conservation and the adoption of green alternatives. Simultaneously, the pharmaceutical industry is witnessing a rise in projects centered around end-customer priorities, such as sustainable packaging and shipping solutions. As supply chains strive to integrate sustainability as a core theme, they are facing mounting pressure to become resilient and cost-effective. This article explores how digital technology can address these challenges sustainably.
What is the business case for enhanced drug forecasting within the clinical development phase?
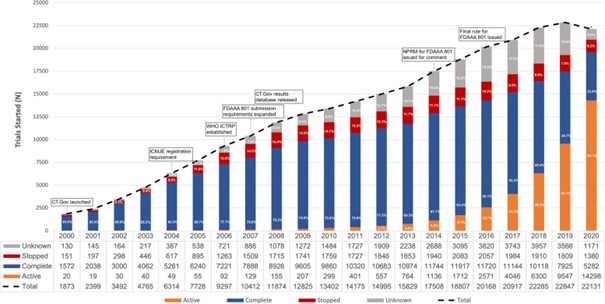
The number of clinical trials has been rising since 2000. At the same time, the projected return on investment of the global pharmaceutical industry went down significantly. This was revealed by Deloitte’s annual analysis of 20 of the world’s leading pharmaceutical companies. The average expected return on investment for research and development fell from 6.8 percent in 2021 to just 1.2 percent in 2022. The average return on investment in research and development was 4.1 percent in 2023. Returns in the previous year had fallen to an all-time low of 1.2 percent. After a dip the year before, 2023 saw the 20 largest pharmaceutical companies invest more again in research into new drugs and therapies. The development process for new drugs must therefore be transformed and could be made much more efficient with digital technologies.1,2
How to make impactful decisions
During clinical development, ensuring the availability of drug products at the right time and place is crucial for adhering to clinical protocols and achieving optimal patient outcomes. Placing the patient at the heart of clinical development necessitates intelligent clinical supply chain design and inventory strategies. Agility should be prioritized to minimize costs and environmental impact while guaranteeing uninterrupted patient dosing. At the same time, maintaining speed of delivery remains key to ensuring innovative therapies are rapidly brought to market.
In the context of a product's life cycle footprint, the design phase holds the most significant influence. This holds true for both medicine and the clinical supply chain, considering their adherence to highly regulated processes. For certain products, production can contribute to up to 80% of their CO2 footprint, as indicated by initial life cycle assessments.3 Within the pharmaceutical industry — a highly regulated industry — implementing design changes is even more complex, so getting the design right from the start becomes even more important.
Additionally, McKinsey reports an alarming average of 50% clinical trial medication waste, highlighting the importance of efficient clinical supply chains to ensure global patient dosing.4
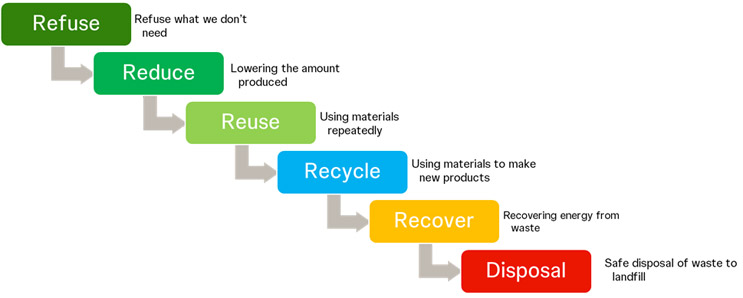
The Clinical Supply Waste Hierarchy
The figure below illustrates the clinical supply chain hierarchy from the perspective of an investigative medicinal product. While efforts and investments in reducing waste through reusable and recyclable materials and shipments are already in progress, the key focus should be on waste reduction in the refuse phase. An excellent example of waste reduction in the clinical supply chain lies in digitalizing the forecast and optimizing inventory and shipments. Meticulous risk assessment, investigational product manufacturing, and overall supply chain process evaluation can drive waste reduction, resulting in cost savings throughout.
Pain points of the current forecasting process
The traditional forecasting process, commonly used in the industry, relies on randomization and trial supply management systems (RTSM) and planning systems that utilize actual data. This analysis work is typically performed by experienced professionals. Forecasts are collected through RTSM systems and compared with forecasts from clinical operations teams. Complex algorithms are then employed to establish an optimal supply plan and determine the required amounts of investigational medicinal products for manufacturing.
However, this approach has its limitations. The inputs to the forecasting system may not provide the necessary granularity for accurate predictions. The factor of unpredictability of patient enrollment remains high. As a result, investigational medicinal products are included in plans but may not end up being used by patients who truly need them, leading to waste at depots or clinical sites.
Enhanced forecasting process
Enhanced forecasting processes take an end-to-end approach. Forecasting at the appropriate level is crucial for maximizing the value within the clinical supply chain. Depending on the design of the supply chain distribution, regional forecasting becomes even more important. In a global context, aligning feasibility, start-up plans, and regional forecasts can improve prediction accuracy and ensure supplies are delivered to the locations where they are most needed. Recent advancements in algorithms and machine learning present opportunities for more accurate local-level predictions and faster responsiveness. The use of artificial intelligence enables real-time enhancements to predictions. However, challenges may arise when integrating systems, particularly in complex supply chains.
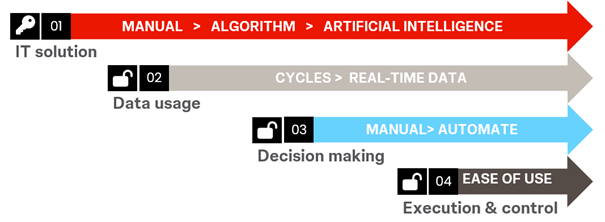
How to make this enhanced forecasting process sustainable
As true for each effective process, the measuring and controlling of performance remains important. Setting KPIs throughout the forecasting process and combining these with established supply chain metrics and bearing in mind the sensitivity and responsiveness of the supply chain is key.
The use of integrated technology will allow a reduction of cycle times, drive the decision-making to more real-time, and enable spotting trends and risks more quickly.
Last is the ease of use and visualization for the end user. Without the ability to understand what value the solution brings throughout the forecast cycle and the automated inputting of data, the quality of the system might not generate the desired outputs for actionability.
Conclusion
Optimizing forecasts through the digitalization of data presents a significant opportunity to enhance reliability, cost-effectiveness, and waste reduction within the clinical supply chain. Evaluating the sustainability impact of these measures is worth considering and incorporating during the design phase to ensure comprehensive business and environmental benefits.
References:
- https://pubmed.ncbi.nlm.nih.gov/36203212/
- https://www2.deloitte.com/ch/en/pages/press-releases/articles/deloitte-pharma-study-drop-off-in-returns-on-r-and-d-investments-sharp-decline-in-peak-sales-per-asset.html, https://www2.deloitte.com/content/dam/Deloitte/ch/Documents/life-sciences-health-care/ch-deloitte-media-release-pharma-innovation-2024-13052024.pdf
- Ellen MacArthur Foundation
- https://www.mckinsey.com/industries/life-sciences/our-insights/clinical-supply chains-how-to-boost-excellence-and-innovation
About The Author:
 Kristel Rens is the director of business transformation and innovation within the clinical supply chain of J&J Innovative Medicine. With over 17 years in various roles within the life sciences industry, she is a well-rounded generalist. Kristel holds expertise in global supply chain management and specializes in creating digital and sustainable innovative solutions for supply chain design.
Kristel Rens is the director of business transformation and innovation within the clinical supply chain of J&J Innovative Medicine. With over 17 years in various roles within the life sciences industry, she is a well-rounded generalist. Kristel holds expertise in global supply chain management and specializes in creating digital and sustainable innovative solutions for supply chain design.
Kristel holds a master’s in chemical engineering and an executive MBA from Antwerp Management School.
