CLINICAL SUPPLY REGULATORY ARTICLES
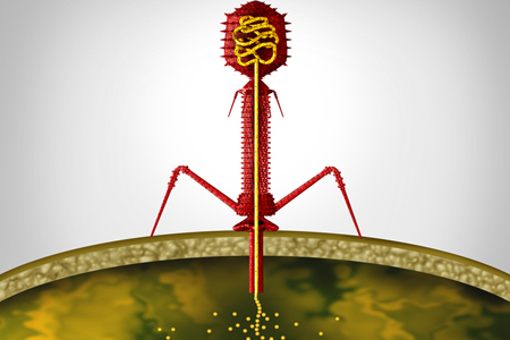 Phages As A Pharmaceutical: New EMA Guidance On Antimicrobial Drug Development
Phages As A Pharmaceutical: New EMA Guidance On Antimicrobial Drug Development
The EMA has now issued a draft guidance on quality aspects of phage therapy medicinal products. Rising antimicrobial resistance has renewed interest in bacteriophages to fight pathogens.
CLINICAL SUPPLY REGULATORY RESOURCES
-
Advanced cGMP facilities deliver control, expertise, and reliable processes that protect quality and support smooth progress to market. Strong partnerships help reduce risk in complex programs.
-
Many small and/or early-stage companies are not aware of the need, or the possibility, of finding a CDMO that specializes in meeting their specific requirements.
-
Analyze the benefits and challenges of implementing direct-to-patient services in the clinical supply chain to help meet patient requirements, improve supply chain efficiency, and accelerate speed to market.
-
Discover the guidance for navigating unique formulation and handling requirements, novel trial designs and supply chain implications, and regulatory and clinical strategies to support product approvals.
-
Start leveraging logistics partners with global end-to-end solutions that can help avoid costly delays, ensure high product quality, and accelerate impactful solutions for patients worldwide.
-
Delve into a solution that ensures the efficient delivery of trial materials worldwide, while navigating the challenges of more complex study designs and the ever-evolving regulatory landscape.
-
Clinical medical writing for gene therapy regulatory documents is a demanding, detail-oriented task that diverges significantly from medical writing for more traditional therapies.