CLINICAL SUPPLY MANUFACTURING ARTICLES
 U.S. Pharma Tariffs And MFN In 2026: Manufacturing And Procurement Impact
U.S. Pharma Tariffs And MFN In 2026: Manufacturing And Procurement Impact
Beroe Inc.'s Mathini Ilancheran discusses the 2025 U.S. pharma tariff framework and translates it into actionable procurement and outsourcing responses.
CLINICAL SUPPLY MANUFACTURING RESOURCES
-
While cell and gene therapies are bringing new hope to patients experiencing rare and serious diseases, uncover how they're disrupting the biopharma market in new and challenging ways.
-
Delve into a solution that ensures the efficient delivery of trial materials worldwide, while navigating the challenges of more complex study designs and the ever-evolving regulatory landscape.
-
Drug discovery companies, CROs, and CDMOs are under pressure to find supply management solutions that ensure delivery of clinical trial materials while navigating complex study designs and the regulatory landscape.
-
Obtaining information and proactively mapping out your fill/finish strategy early can de-risk an investment in equipment or processes, ensuring you meet your long-term needs.
-
Advanced cGMP facilities deliver control, expertise, and reliable processes that protect quality and support smooth progress to market. Strong partnerships help reduce risk in complex programs.
-
Handling development and manufacturing challenges for orphan drugs requires experience, expertise and scalable technologies, along with a flexible and agile supply chain.
-
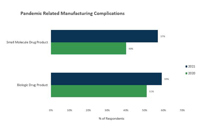
Find out how COVID-19 complications have picked up from the first year, with more outsourcers experiencing bigger obstacles when it comes to manufacturing, specifically with drug product.