Global Clinical Supply Chain in Emerging Markets: Reliability, Compliance, Access
By Rakib Ouro-Djobo, Global Clinical Supply Chain Leader

Emerging markets, particularly in Africa, Asia, and South America, are increasingly vital to global drug development, offering epidemiological diversity and access to over 2.5 billion people. Despite this potential, these regions host a disproportionately low number of clinical trials, with Africa accounting for only 1.1% of global activity.1,2 The primary barrier is not scientific but systemic: the fragility of the clinical supply chain.
In high-complexity regions, clinical supply must be reframed from a traditional logistics function into a systems design challenge. Reliability, compliance, and access are not independent considerations but interdependent design requirements that determine whether the investigational product ultimately reaches the patient and protects trial integrity.
The need for diverse patient populations and the growing burden of disease in these regions necessitate their central role in clinical research. However, applying supply chain models developed for mature Western infrastructure inevitably leads to failure, cost overruns, and compromised data integrity. Success requires a fundamental shift in design philosophy: one that assumes variability and engineers resilience. For clinical supply leaders, this shift fundamentally changes how supply chains must be designed, qualified, and governed across high-variability regions.
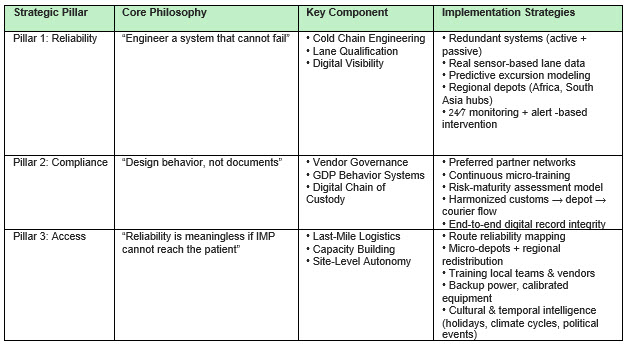
Engineering Reliability — From Fragile To Predictable
Reliability in emerging markets is not achieved through standard procedures but through redundancy and predictive engineering. The core philosophy must be to engineer a system that cannot fail, recognizing the inherent constraints of the operating environment.
The primary threats to product integrity are unstable power grids, inconsistent lane performance, and extreme ambient temperatures (often exceeding 35 to 45 degrees C), which drastically reduce the thermal buffer time for temperature-sensitive investigational medicinal products (IMP).
The Engineering Solution
The strategic response is a move toward a highly visible and actively managed cold chain:
- Dual-Redundancy Cold Chain: For high-value IMP, the deployment of both active (powered) and passive (insulated) packaging systems is mandatory. This provides a critical safety net during power failures or prolonged transit delays.
- Lane Qualification via Real-Time Data: Qualification must move beyond vendor assurances to utilize historical sensor-based data for every specific corridor. This enables predictive excursion modeling, in which the optimal packaging stock keeping unit (SKU) is selected based on the lane’s historical risk profile, accounting for seasonal and political variations.
- Regional Buffer Depots: Establishing strategic hubs in major regional centers (e.g., Nairobi, Johannesburg, Mumbai, Singapore) shortens the final distance between storage and the clinical site. This strategy reduces transit time, isolates failures, and minimizes exposure to high-risk customs or long-haul routes.3
- 24/7 Visibility and Alert-Based Intervention: IoT-enabled monitoring systems must provide continuous real-time temperature and location data. The system’s value lies not just in documentation but in triggering automated alerts that enable intervention before a temperature excursion occurs.
Designing Compliance — A Behavioral Ecosystem
Good manufacturing practice (GMP) and good distribution practice (GDP) compliance in emerging markets is less about the volume of documentation and more about the design of behavioral systems. The principle is to design behavior, not documents, ensuring that the correct procedure is the path of least resistance. This approach reduces dependency on manual oversight and lowers the risk of supply interruptions caused by human error, inconsistent training, or incomplete documentation.
Navigating Regulatory Complexity
The regulatory landscape is fragmented, though significant harmonization efforts are underway.
- African Harmonization: Initiatives like the African Medicines Agency (AMA) and regional blocs such as the East African Community (EAC) Medicines Regulatory Harmonization (MRH) are working to unify disparate national frameworks.4 However, sponsors must still navigate individual national regulatory authorities (NRAs) and their distinct requirements.
- Indian Regulatory Framework: In India, the New Drugs and Clinical Trials Rules (2019) have introduced a more streamlined process. However, the critical supply chain bottleneck remains the precise navigation of the CDSCO SUGAM portal for import permissions, specifically Form CT-16 (application) and Form CT-17 (import license).5 Customs bottlenecks, often exacerbated by inconsistent documentation, can turn a 48-hour shipment into a multi-week delay.
Engineering Compliance
- Continuous Micro-Learning: Compliance training must be embedded into daily workflows. Digital platforms should deliver bite-size, role-specific GDP refreshers, rather than ineffective one-time onboarding events.
- Standardized Vendor Governance: A rigorous audit-driven process is necessary to select preferred partners (couriers, customs brokers, depots) who demonstrate a deep, consistent understanding of GDP and local regulatory nuances.
- Digital Chain of Custody: Reliance on paper records or spreadsheets for chain-of-custody, temperature logs, or accountability is a critical failure point. End-to-end digital platforms are essential to ensure data integrity, audit readiness, and immediate accountability.
Unlocking Access — The Last-Mile Imperative
The final 5% of the supply chain — the last mile — is where trials are ultimately won or lost. The core philosophy here is that reliability is meaningless if IMP cannot reach the patient. Last-mile success is a patient-centric reliability question that extends beyond transportation to encompass site-level autonomy. In clinical trials, access is ultimately a supply chain outcome: IMP availability at the right site, at the right time, in the right condition.
Site-Level Vulnerabilities
Common failure points at the site level include:
- Infrastructure Failure: Inadequate backup power, uncalibrated or failing storage equipment, and a lack of trained personnel.
- Temporal and Cultural Intelligence: Deliveries must account for local holidays, religious observances, climate cycles (e.g., monsoon seasons), and potential political disruptions.
- Staff Turnover: High turnover at clinical sites necessitates continuous automated training to maintain GDP awareness.
A Better Blueprint
- Pre-Activation Infrastructure Assessments: Rigorous audits of site-level equipment (freezers, generators) and personnel training are nonnegotiable prerequisites for site activation.
- Micro-Depots and Regional Redistribution: Utilizing smaller localized distribution centers cuts dependency on unstable long-haul lanes and allows for faster, more flexible response to site demand.
- Capacity Building as Strategy: Investing in local teams, vendors, and infrastructure is not merely a corporate social responsibility (CSR) activity; it directly improves supply reliability, reduces resupply lead times, and lowers total landed cost over the life of a trial.6
- On-Site Autonomy: Empowering sites with calibrated equipment, backup power solutions, and real-time monitoring allows them to manage their own inventory with minimal external dependency, thereby increasing resilience.
Digital Visibility: The New Currency Of Trust
In the high-stakes environment of emerging markets, you cannot manage what you cannot see. For clinical supply teams, visibility is what enables proactive intervention before excursions, stockouts, or site disruptions impact patients. A modern clinical supply chain requires a digital nervous system that integrates data from multiple sources to enable predictive operations.
The Integrated Digital Nervous System
The system must integrate:
- Real-Time Data: Continuous monitoring of temperature, location, and customs status from manufacturer to patient.
- AI-Driven Risk Scoring: Machine learning models should predict potential delays or excursions based on weather, traffic, and historical performance, allowing for proactive mitigation.
- Centralized Dashboards: A single source of truth for vendor performance, interactive response technology (IRT) reconciliation, site inventory alignment, and patient demand forecasting.
This integration shifts the supply chain function from reactive firefighting to predictive operations, significantly reducing waste and enhancing patient safety.
The Emerging Market Supply Chain Equation
The strategic design of clinical supply chains for emerging markets can be summarized by a simple, yet powerful, equation.
Global Clinical Supply Chain Design for Emerging Markets
Reliability + Compliance + Access = Patient-Centric Delivery
For clinical supply leaders operating in emerging markets, this equation provides a practical design lens for balancing risk, cost, and patient impact across increasingly complex global trials.
This framework is the blueprint for success in high-complexity regions. Sponsors who move away from Western assumptions and embrace this design-first approach will not only accelerate development and protect product integrity but will also ensure equitable access to lifesaving therapies. The future belongs to organizations that treat their supply chains not as cost centers, but as strategic assets that unlock global patient access.
References:
- bioxconomy.com. Africa clinical trials face logistics challenges. [URL: https://www.bioxconomy.com/clinical-and-research/africas-clinical-trial-expansion-hindered-by-logistics-challenges-despite-growing-population]
- weforum.org. Clinical trials are key to improving medicines access in Africa. [URL: https://www.weforum.org/stories/2025/07/healthcare-access-africa-clinical-trials/]
- lslog.com. Future-Proof Your Cold Chain for Resilient Clinical and.... [URL: https://www.lslog.com/future-proof-your-healthcare-cold-chain/]
- celegence.com. Regulatory Approvals in Africa: Key Frameworks, Challenges, and Emerging Trends. [URL: https://www.celegence.com/regulatory-approvals-in-africa-key-frameworks-challenges-and-emerging-trends/]
- nsws.gov.in. CT-16 - APPLICATION FOR GRANT OF LICENCE TO.... [URL: https://www.nsws.gov.in/portal/approval-details/ministry-of-health-and-family-welfare/directorate-general-of-health-services/ct-16-application-for-grant-of-licence-to-import-new-drug-or-investigational-new-drug-for-clinical-trial-or-bioavailability-or-bioequivalence-study-or-for-examination-test-and-analysis]
- gavi.org. How scaling up clinical research in Africa can benefit society and economy. [URL: https://www.gavi.org/vaccineswork/how-scaling-clinical-research-africa-can-benefit-society-and-economy]
About the Author:
 Rakib Ouro-Djobo is a global supply chain executive redefining how life-science organizations achieve clinical and commercial readiness. With more than 15 years of experience across small molecules, biologics, and advanced therapies, he is known for building resilient, launch-ready supply ecosystems that accelerate trials, reduce risk, and expand patient access worldwide. Rakib operates at the intersection of strategy and execution — helping biotechs and stakeholders scale with confidence through their most pivotal growth moments.
Rakib Ouro-Djobo is a global supply chain executive redefining how life-science organizations achieve clinical and commercial readiness. With more than 15 years of experience across small molecules, biologics, and advanced therapies, he is known for building resilient, launch-ready supply ecosystems that accelerate trials, reduce risk, and expand patient access worldwide. Rakib operates at the intersection of strategy and execution — helping biotechs and stakeholders scale with confidence through their most pivotal growth moments.
