Making Cancer Vaccines Is Complex; New Platform Guidance Could Help
By Antony Hitchcock, principal and owner, AGH Bioconsulting

The much-awaited FDA draft guidance on platform processes, titled Platform Technology Designation Program for Drug Development, offers the potential for simplified and accelerated product registrations and includes specific reference to emerging technology including mRNA and LNPs as product areas where platform processes may be applied for.
Clearly, the draft guidance has been developed largely around the products made through classic large-scale batch-mode production. What I want to explore here is how the draft guidance might affect another emerging area of medicine — personalized medicine, specifically, cancer treatment.
Historically, biologic medicines have been produced in batch-mode processes. Increased demand has been addressed by increasing batch sizes and number. This has been true of both small molecule and biological products such as monoclonal antibodies (mAbs). However, personalized therapeutic cancer vaccines also meet the platform process criteria. As we gain increased knowledge of different diseases at a genetic and individual level, alongside the potential for developing treatment approaches tailored to specific patient needs, there has been a drive to develop personalized treatment approaches, notably in oncology.
The production of personalized therapies presents a very different manufacturing challenge compared to conventional therapeutic products. As I will discuss here, they are unique to individual patients, but their manufacture may benefit from the use of platform processes.
Defining Personalized Therapies
A key area for personalized therapies has been the production of autologous cell therapies such as CAR-T, where patients’ cells are removed, purified, and then genetically modified to train the cells to identify and attack a patient’s cancerous blood cells.
One of the key challenges for these products has been logistics. Manufacturers must produce, test, and release product within very restricted timelines. Successfully producing these products requires robust production and testing platforms, as these products give no scope for process optimization or, critically, for batch failure. While these products have brought significant benefit to patients to date, their use has been limited. CAR-T therapies are approved only for blood-borne cancers, and their cost is exorbitant.
Cancer Vaccines
CAR-Ts, which are just one approach to immunotherapy, recently have led the emergence of a new immune-based treatment approach in the form of cancer vaccines, which train the immune system to identify multiple tumor-specific antigens (TSAs). Rather than a singular treatment, patients are dosed with these products over 12 to 18 months in combination with checkpoint inhibitors (CPIs). Some products have been shown in clinical studies to further improve cancer survival rates.
These vaccines take a wide range of formats, and while initial studies were based on peptide vaccines, more recent developments have focused on mRNA and plasmid DNA formats and, in some cases, viral vectors.
There are two approaches to cancer vaccines. The first is “off-the-shelf,” in which vaccines are generated containing TSAs identified from multiple patient tumors of similar cancer types. The second approach is to generate a vaccine against antigens identified from an individual patient’s tumor. While this second approach generates a significant level of complexity, from a clinical perspective, the personalized approach is seen as a way of preventing mutational escape, which can occur as selective pressure is placed on tumors by the immune system.
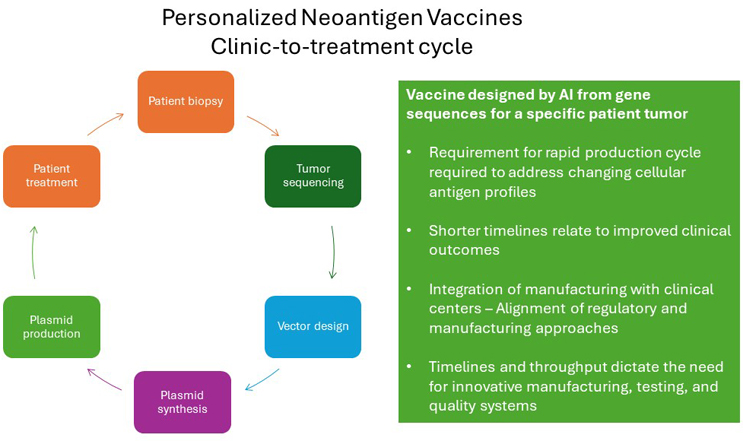
Speed to treatment is a critical factor for any cancer patient. Consequently, a key challenge to producing personalized therapies has been manufacturing timelines, including the testing and release of products ahead of administration to patients. This requirement has challenged norms not just with manufacturing. It also highlights the need for accelerated testing procedures and product release.
A second challenge will be throughput. Many of these products target solid tumors, which have larger patient populations than blood-borne cancers.
Product Design And Production
A unique element of personalized vaccines is the design system, which is based on outputs from the sequencing of tumor DNA to identify tumor antigens and assembling these antigens into a vaccine construct using AI systems. These platforms tend to be unique to the platform developer and are a key element of their technology.
The most common formats are mRNA and plasmid DNA, which include standard backbone sequences with unique sequences coding for the specific vaccine antigens. The reason to use mRNA and plasmid vaccines is that they can deliver multiple antigens, which is a key requirement for these products. They also can be produced using platform processes.
The platform is a critical requirement, as the level of throughput and timelines gives no opportunities for process optimization or introducing process changes. The process must be resistant to both changes in gene sequence and to failure, as production delays are likely to have a serious effect on patient outcomes.
It is not surprising that, to address these challenges, several manufacturers have adopted cell-free platforms. While these platforms still require a plasmid DNA template, their product relies on a series of standardized enzyme-mediated chemical reactions followed by a standardized chromatographic purification step. This also applies to drug product manufacturing, which may include formulations such as lipid nanoparticles or naked injections.
Testing And Release
Testing and release of personalized therapies pose significant challenges regarding throughput and timelines. Here, manufacturers need innovative testing procedures, including rapid sterility testing, and procedures that allow for simultaneous testing of multiple batches.
Quality Systems
To achieve the required levels of process consistency and throughput, extensive quality systems are imperative. The adoption of quality by design, including for raw materials and the process enzymes used, is necessary to control individual process steps through online measurements and control. Considering the levels of throughput, it is likely that product release must be completed by exception rather than by a detailed review of batch documents.
How The Platform Guidance Applies To Personalized Therapies
When looking at this in light of the draft FDA platform guidance, many of the production elements used to make these products are likely to meet the requirements for platform processes. The most obvious elements are the production of vaccine drug substance and drug product, especially for mRNA vectors, which the guidance specifically highlights.
A key question is, how far can the process go?
An interesting comment within the draft guidance relates to the potential reduction in validation and analytical testing for products. This is important for personalized vaccines because testing is a significant burden in terms of cost per product, so any potential to reduce testing is likely to be welcome. The question will be how far regulators will go in this area.
Unlike conventional therapeutics, producers will be producing hundreds if not thousands of cancer vaccine batches even before product registration, so it is likely that a vast amount of data will be generated around the process performance. This information will relate to process robustness and the product’s ability to produce different antigen sequences and process outcomes in terms of product purity and process performance, which, in turn, should provide detailed information on critical process parameters and operational ranges required to achieve desired outcomes.
Given the significant amount of data collected, we should ask whether validation of these processes should be required at all.
It is likely that production processes will be highly automated with on-line analysis and control based on the known critical process parameters. The question then arises that if processes are sufficiently under control and shown to be robust with regard to vaccine design, what is required when considering final product testing? What value is added compared to the potential benefits of patients receiving early access to the treatment and the potential reduction in cost?
Evolving Technologies
The final element that could be regarded as a platform is the product design. This presents a more challenging question. Will the design processes based around AI technologies be regarded as platform technologies that can be applied to multiple products? Regulators will have to address that in the future.
Personalized therapies are a potential game-changer for treating deadly disease, but they challenge established thinking and regulations. It’s unclear now how the guidance addresses rapidly evolving technologies and treatment approaches. The guidance acknowledges historical process data to be used to support license applications, but what is not so clear is how close these platforms need to match those historical steps and how much latitude is allowed for innovation and development.
Not only are these products themselves highly innovative, but so are the processes used to produce them.
It is possible that the equipment and platforms used in early clinical development could become outdated before the product is licensed.
Production procedures will inevitably evolve rapidly over the coming years, not least with regard to levels of automation, process control, and, potentially, key elements of the manufacturing process. This evolution brings up a potentially serious concern around the proposed regulations, namely, how will regulators deal with issues around innovation and change and, critically, what level of change will disqualify processes from being platforms? Will this guidance benefit manufacturers, or could it be a potential barrier to companies looking to innovate and build onto established platforms?
Going forward, it is essential in the application of this guidance that regulators give scope and support for innovation and development of platform processes whether it be for mAbs or cancer vaccines.
However, if it is perceived that changes to platforms may potentially delay or adversely impact product registrations, then there is the potential to disincentivize innovation and stymie investments in platform improvements.
Conclusions
The draft guidance comes at a time when we are seeing significant changes in the clinical approaches to the treatment of many diseases. Increased knowledge and development in manufacturing technologies allow us to produce truly personalized therapies, which could lead to significant improvements in patient outcomes.
To ensure that patients benefit from these developments, there is a need for proposed and future guidance to acknowledge and support these highly innovative new medicines and clinical approaches.
About The Author:
 Tony Hitchcock is the principal and owner of AGH Bioconsulting. He has spent his decades-long career in the in the biotechnology field, with over 30 years in the production of complex biologic for clinical trials in the European Union and U.S. He has worked in areas of process development and manufacturing with experience in engineering and process systems. He has worked on the development of more than 30 products for clinical trials, including plasmid DNA, viral and bacteriophage products, and recombinant proteins from microbial, mammalian, and insect cell sources. Contact him at tony@aghbioconsulting.com.
Tony Hitchcock is the principal and owner of AGH Bioconsulting. He has spent his decades-long career in the in the biotechnology field, with over 30 years in the production of complex biologic for clinical trials in the European Union and U.S. He has worked in areas of process development and manufacturing with experience in engineering and process systems. He has worked on the development of more than 30 products for clinical trials, including plasmid DNA, viral and bacteriophage products, and recombinant proteins from microbial, mammalian, and insect cell sources. Contact him at tony@aghbioconsulting.com.
