3 Strategies For mAb Manufacturing: How Do You Choose?
By Chun Chen, Bill & Melinda Gates Medical Research Institute
Monoclonal antibodies (mAbs) are now used to treat different types of cancer, autoimmune disorders, and infectious diseases like RSV and COVID-19. Although mAbs have demonstrated success in treating various conditions, the high cost of these therapies has limited their wide-scale use. This impact is felt most by patients in low- and middle-income countries (LMICs).
A major cost driver in making mAbs is manufacturing capacity. Building or expanding in-house capacity can require substantial at-risk capital investment, with uncertainties in commercial demand and regulatory approval. As cost and supply come under more scrutiny, various manufacturing options (in-house vs. contract manufacturing) and different scaling strategies have surfaced as opportunities to improve efficiency and, thus, reduce the cost. For mass production to meet patient demand, there are primarily three scaling strategies for mAb manufacturing that can be leveraged, each with its own set of pros and cons: scale out, scale up, and scale by time.
A Comparison of Strategies
To improve output and efficiency, mAb manufacturers should select an approach only after carefully considering key factors of interest for their specific program needs, including cost, speed, flexibility, and more. The below image, Figure 1, illustrates the pros and cons of the three strategies.
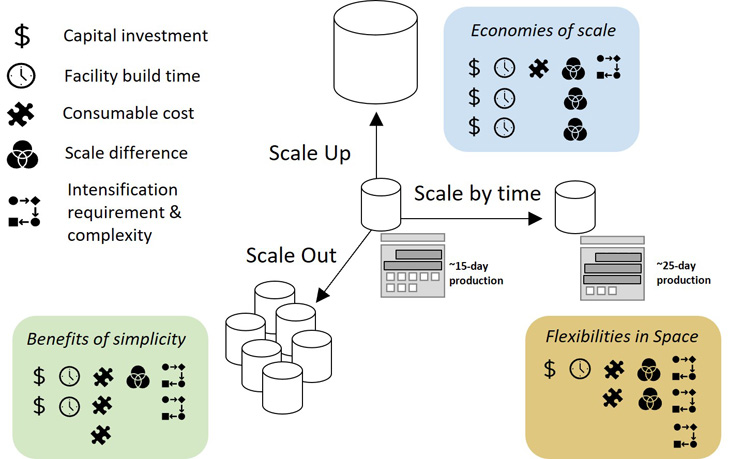
Figure 1: Biomanufacturing formats and scaling up strategies. A comparison of strategies to scale up commercial manufacturing of mAbs utilizing three different dimensions: scale up to, i.e., increasing the size of the product tank; scale out, i.e., increasing the number of product tanks; and scale by time, i.e., extending the time or duration of the tank operation. The comparison considers the advantages and disadvantages of each regarding key factors such as capital investment requirement, facility build time, consumable cost, scale differences between development and commercial stages, and process complexity. Different symbols are used to denote each factor, and the number of symbols next to the format is used to denote how that factor adversely impacts the format. One symbol: low impact; two symbols: medium impact; three symbols: high impact. Figure adapted from Chen C. et al. Cost and supply considerations for antibody therapeutics Mabs. (2025), published under CC BY-NC.
1. Scale Up
The scaling up strategy calls for the use of stainless steel tanks for cell culture fermentation that hold more than 12,000 liters. The benefit of this approach is that manufacturers can attain economies of scale, which is easier to achieve with products that have high demand. The downside of this approach is higher up-front capital investment and more facility build time and space (the larger tanks and supporting infrastructure require more facility space). Also, there can be significant operational costs associated with cleaning and sterilizing large tanks that must be taken into consideration when determining the overall financial impact of this strategy.
2. Scale Out
This strategy involves increasing the number of bioreactors used for mAb production. Diverging from the traditional format that employs the large stainless steel tanks, the scale-out approach pivots instead toward smaller single-use bioreactor tanks, which hold about 2,000-6,000 liters for cell culture fermentation. Some of the benefits of this strategy include less up-front capital investment compared to scaling up and shorter facility build time. For commercialization, rather than scaling up from smaller to larger tanks to meet high demand, facilities can simply scale out by adding identical tanks or building new facilities. This strategy also can mitigate manufacturing risks. In the event of a tainted batch, only a small tank would be affected — rather than a larger, more expensive tank used by the scale-up strategy.
The downside to this approach is that operating more tanks and facilities can add layers of complexity and complications. It also requires more expenses on materials due to the increase in single-use consumables, so it is not necessarily more economical than the scale-up option.
3. Scale By Time
The scaling by time strategy relies on extending the duration of bioreactor operation instead of using larger or more bioreactors to increase output. This tactic ― the most recent trend in biomanufacturing ― further reduces factory footprint, build time, and facility-related costs compared to the scale-out strategy by leveraging the latest process intensification and continuous manufacturing technologies, which typically extends the duration of the cell culture process to more than two weeks. This approach also minimizes downtime of the facility by removing the “white space” required for equipment turnaround between manufacturing processes. However, if the batch size achievable through this approach is not large enough, relatively higher overhead costs for maintaining equipment, facility, and other batch-specific costs may offset any benefits of this tactic. Moreover, the operational complexity, the time and resources needed for process development should also be considered before adopting this approach for better economics.
The Importance of Ongoing Analysis
For products with specific price targets or supply pressures, it’s important that manufacturers conduct cost and supply assessments throughout the product life cycle, from beginning to end. Early and ongoing economic analysis of cost of goods (COGs) is important to ensure the viability of the proposed drug, especially for products developed for LMICs. The ongoing analysis also will help guide process development and decision-making throughout the product development life cycle, e.g., choosing the right manufacturing format and scaling strategy.
There are several software applications that can help manufacturers better understand and plan for the various factors that impact the economics of mAb manufacturing (Figure 2). Manufacturers can use these tools to evaluate and improve a wide variety of factors, including those associated with facilities, processes, or the products themselves.
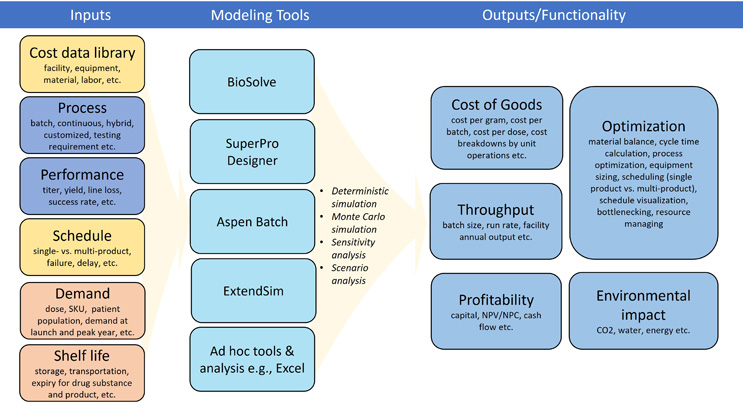
Figure 2: Summary of existing process economic analysis tools. The column on the left: modeling inputs, with yellow boxes representing facility-related inputs, blue boxes representing process-related inputs, and red boxes representing product-related inputs. The column in the middle: various modeling tools. The column on the right: outputs and functionalities of various modeling tools. Figure adapted from Chen C. et al. Cost and supply considerations for antibody therapeutics Mabs. (2025), published under CC BY-NC.
One resource, based on the Microsoft Excel program, is a process simulator featuring a database that analyzes costs associated with the facility, the process, the equipment being used, and the cost of raw materials and labor. This sort of tool, which can be customized with specific data sets, allows users to contrast different manufacturing strategies, creating a side-by-side comparison with detailed cost, supply, and environmental implications of each element in the analysis.
Final Thoughts
In closing, pharmaceutical manufacturers must remain efficient, agile, and flexible to survive in an increasingly competitive industry — and to realize the full potential of mAbs to tackle the disease burden in LMICs.
For the malaria mAb under development at the Bill & Melinda Gates Medical Research Institute, ongoing cost and supply analysis has been adopted to guide decisions throughout a cost-conscious process development for manufacturing, e.g., selecting a scaling strategy best fits the program needs. If we can successfully develop and manufacture a cost-effective malaria mAb for LMICs, the same model can be applied to lower the costs of other mAbs that could benefit LMICs, thus improving profitability and/or affordability in high-income countries (HICs) as well. We recognize that the best manufacturing process and strategy will ultimately depend on the specific circumstances of the product being produced, as cost sometimes can be a lower priority than other factors, e.g., speed-to-clinic.
With all that we know today, mAb development organizations and manufacturers have the knowledge, technologies, and experience ready to proactively serve LMICs markets. With process innovations and cost-saving measures, producing affordable mAbs is not just possible, it is vitally important for global health. Certainly, mAbs can and should be developed for HICs, but with the technologies and tools now at our disposal, it’s time to rethink LMICs not as just viable markets, but as untapped markets with substantially unrealized potential.
About The Author:
 Chun Chen is a process development and manufacturing lead at the Bill & Melinda Gates Medical Research Institute (Gates MRI). He works closely with research, development, and manufacturing partners to advance the institute’s diverse vaccine and biologics programs. Before joining the institute, Chen spent nine years in Amgen’s Process Development Department, where he led a group to develop drug substance processes for large molecules, focusing on process intensification. Chen also used systems biology, omics, and other data modeling techniques for cell line and process optimization. Chen earned a Ph.D. in genetics, bioinformatics, and computational biology from Virginia Tech.
Chun Chen is a process development and manufacturing lead at the Bill & Melinda Gates Medical Research Institute (Gates MRI). He works closely with research, development, and manufacturing partners to advance the institute’s diverse vaccine and biologics programs. Before joining the institute, Chen spent nine years in Amgen’s Process Development Department, where he led a group to develop drug substance processes for large molecules, focusing on process intensification. Chen also used systems biology, omics, and other data modeling techniques for cell line and process optimization. Chen earned a Ph.D. in genetics, bioinformatics, and computational biology from Virginia Tech.
